Graphite and Diamond are allotropes of carbon ,meaning any of two or more physical forms in which an element can exit. Diamond is very strong in fact strongest known where as graphite is very soft. Why ?
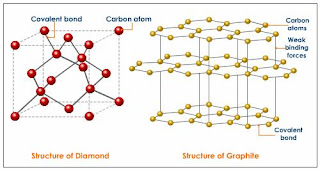
Let us look at the electronic configuration of carbon, it has 4 valence electrons and each carbon atom is covalently bonded to four other carbon atoms in case of diamond.The crystal structure of a diamond is a face-centered cubic or FCC lattice. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).
Diamond's strength comes from pyramid-like structure which is the strongest structures known to man, This is a giant covalent structure and it continues on and on in three dimensions. It is not a molecule, because the number of atoms joined up in a real diamond is completely variable depending on the size of the crystal. As all the four electrons form covalent bonds, there are no free electrons or ions in diamond, so it does not conduct electricity. It has very high melting point (almost 4000°C) because very high temperatures are required to break the covalent bonds. Each carbon atom is at the same distance to each of its neighbouring carbon atoms. In this rigid network atoms cannot move. This explains why diamonds are so hard and have such a high melting point.
Graphite has a layer structure, each carbon atom uses three of its electrons to form simple bonds to its three close neighbours. The fourth electron is free and no longer associated directly with any particular atom or pair of atoms meaning delocalized, but are free to wander throughout the whole sheet.The distance between the layers is about 2.5 times the distance between the atoms within each layer. A weak Van der Waals force of attraction acts between the layers and these layers of carbon slide over each other in graphite, that's why it is very soft and conducts electricity.
No comments:
Post a Comment